| CONTINUE TO |
|---|
| [PART I] Chondrites |
| [PART II] Achondrites |
| [PART III] Irons |
| [PART IV] Stony-Irons |
| [APPENDECTOMY] |
| ORDINARY CHONDRITE CHONDRULE SIZES (µm) after K. Metzler (2018) |
||||
|---|---|---|---|---|
| Mean 2D (3D) |
Median 2D (3D) |
Min 2D (3D) |
Max 2D (3D) |
|
| H4 (NWA 2465) | 450 (490) | 370 (420) | 95 (90) | 5400 (2360) |
| L4 (Saratov) | 500 (610) | 450 (530) | 130 (180) | 2160 (2520) |
| LL4 (NWA 7545) | 690 (830) | 580 (730) | 190 (245) | 3810 (2880) |
| after D. W. Hughes (1978) | ||||
| L/LL4 (Bjurböle) | — (750) | — (688) | 200 (250) | — (—) |
| CO3 Petrographic Types after Sears et al., 1991 |
|||||
|---|---|---|---|---|---|
| TL sens. (120°C) | |||||
| 3.0 | <0.017 | ||||
| 3.1 | 0.017–0.030 | ||||
| 3.2 | 0.030–0.054 | ||||
| 3.3 | 0.054–0.100 | ||||
| 3.4 | 0.100–0.170 | ||||
| 3.5 | 0.170–0.300 | ||||
| 3.6 | 0.300–0.540 | ||||
| 3.7 | 0.540–1.000 | ||||
| 3.8 | 1.000–1.700 | ||||
| 3.9 | >1.700 | ||||
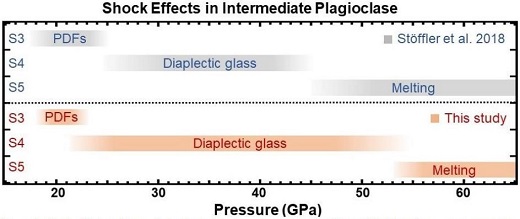
| MINERAL COMPOSITIONS | |||||
|---|---|---|---|---|---|
| *Fe/Mn (pyx) | plag. | Fe-metal | sulfide | secondary alter. | |
| HED | 20––40 | An90 | Y | Troilite | none |
| Angrites | 60&––90 | An99 | N | Troilite | none |
| Moon | 70 | An92 | Y | Troilite | none |
| Earth | 60 | An50 | N | Pyrrh. | hydrous phases |
| Mars | 35 | An50 | N | Pyrrh. | hydrous phases |
| Venus | 55 | — | N | Pyrrh. | anhydrous phases |
| BULK CHEMICAL COMPOSITIONS | ||||
|---|---|---|---|---|
| Fe/Mn | K/U | K/La (×CI) | Rb/La (×CI) | |
| HED | 30 (±2) | 2,000 | 0.03 | 0.002–0.02 |
| Ibitira | 34–36 | — | — | — |
| Angrites | 80–95 | 150 | 0.002–0.03 | 0.001 |
| Moon | 62 (±18); 67 (±9) | 1,700 | 0.03 | 0.016 |
| Earth | 40 (±11) | 12,500 | 0.15 | 0.09 |
| Mars | 35–50 | 15,000 | 0.2 | 0.3 |
| Venus | 55 (±30) | 12,500 | 0.1–0.2 | 0.1 |
| *O-ISOTOPIC COMPOSITIONS | |
|---|---|
| Δ17O (‰) | |
| CR chondrites | –0.96 to –2.42 |
| Lodranites | –0.85 to –1.49 |
| Acapulcoites | –0.85 to –1.22 |
| Winonaites | ~ –0.4 to –0.80 |
| HED | –0.24 |
| Brachinites | –0.13 to –0.30 |
| Angrites | –0.125 |
| Moon | 0.00 |
| Earth | 0.00 |
| Mars | +0.3 |
| Venus | +0.0 to +0.3 |
| H (4–6) | +0.73 |
| L (4–6) | +1.07 |
| LL (4–6) | +1.26 |
| TOPICAL, CHEMICAL, AND ISOTOPIC PARAMETERS | |
|---|---|
| Surface | Depth |
| low formation pressure (~10–25 bars) | high formation pressure (> ~100–125 bars) |
| highly reduced (smelting) | little reduced (smelting suppressed) |
| low δ18O & Δ17O | high δ18O & Δ17O |
| low C content | high C content |
| magnesian (high Mg#; ~75–95) | ferroan (low Mg#; ~60–90) |
| albitic melt clasts (<An25) | labradoritic melt clasts (An39–58) ol–aug melt clasts (An39–54) |
| ol–pig ureilite residues | ol–aug ureilite residues |
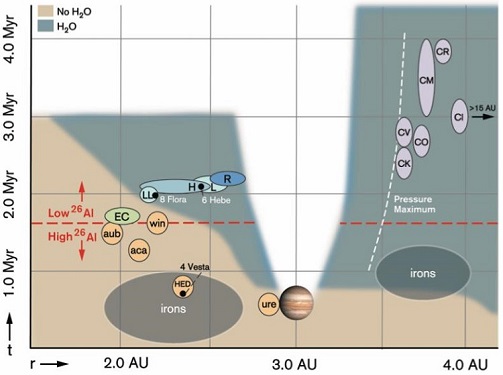
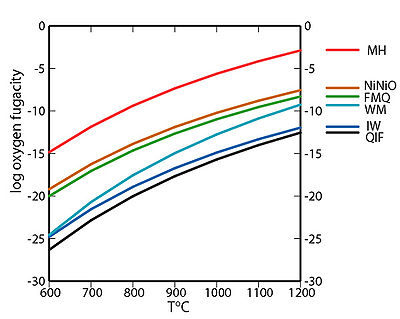
| SOME SHORT- AND LONG-LIVED CHRONOMETERS Norris et al., 1983; Hibiya et al., 2014; Pravdivtseva et al., 2016; Villa et al., 2020, Desch et al., 2022 [I, II] and ref. therein |
||
|---|---|---|
| Parent → Daughter | Half-life | Minerals dated |
| 41Ca → 41K | 0.0994 (±0.0015) m.y. | |
| 36Cl → 36Ar, 36S | 0.301 (±0.002) m.y. | |
| 26Al → 26Mg | 0.717 m.y. (weighted mean) or 0.708 (±0.017) m.y. (Nishiizumi, 2004) or 0.705 m.y. (AMS standard) |
Plag – Olv,Pyx |
| 10Be → 10B | 1.387 (±0.012) m.y. | CAIs, (FUN) CAIs, PLACs |
| 135Cs → 135B | 2.3 (±0.3) m.y. | |
| 60Fe → 60Ni | 2.62 (±0.04) m.y. | Chr – metal |
| 53Mn → 53Cr | 3.98 (±0.11) m.y. | Olv – Chr |
| 107Pd → 107Ag | 6.5 (±0.3) m.y. | metal – metal |
| 182Hf → 182W | 8.896 (±0.089) m.y. | Pyx – metal |
| 247Cm → 235U | 15.6 (±0.5) m.y. | |
| 129I → 129Xe | 16.14 (±0.12) m.y. | Pyx – Pyx |
| 205Pb → 205Tl | 17.3 (±0.7) m.y. | |
| 92Nb → 92Zr | 34.7 (±0.7) m.y. | Pyx – Chr |
| 146Sm → 142Nd | *68 (±7) m.y. or **103 (±5) m.y. |
Chr,Pyx – Plag |
| 244Pu → 236U, 232Th | 80.0 (±0.9) m.y. | |
| 235U → 207Pb | 703.81 (±0.96) m.y. | Zircon – Zircon |
| 176Lu → 176Hf | 3.54 b.y. | Pyx – Chr |
| 238U → 206Pb | 4.4683 (±0.0048) b.y. | Zircon – Zircon |
| 87Rb → 87Sr | 49.61 (±0.16) b.y. | metal – Plag |
| 147Sm → 143Nd | *106.25 (±0.38) b.y. | Chr,Pyx – Plag |
| CONTINUE TO |
|---|
| [PART I] Chondrites |
| [PART II] Achondrites |
| [PART III] Irons |
| [PART IV] Stony-Irons |
| [APPENDECTOMY] |